Introduction
Matter changes all around us because of a basic process called a chemical reaction. Double displacement reactions are different from other types of chemical reactions because they have unique properties and can be used in many different ways. In this complete guide, we will learn about double displacement reactions, including what they are, how they work, some examples, and what they mean in different areas of chemistry.
How to Figure Out Chemical Reactions
1.1 Chemical Reactions: How They Work
Chemical reactions involve the transformation of substances into new substances with different properties. This section provides a brief overview of the fundamental concepts of chemical reactions.
1.2 Chemical Reactions and Their Types
Explore the various types of chemical reactions, such as synthesis, decomposition, combustion, and more, to set the stage for understanding double displacement reactions.
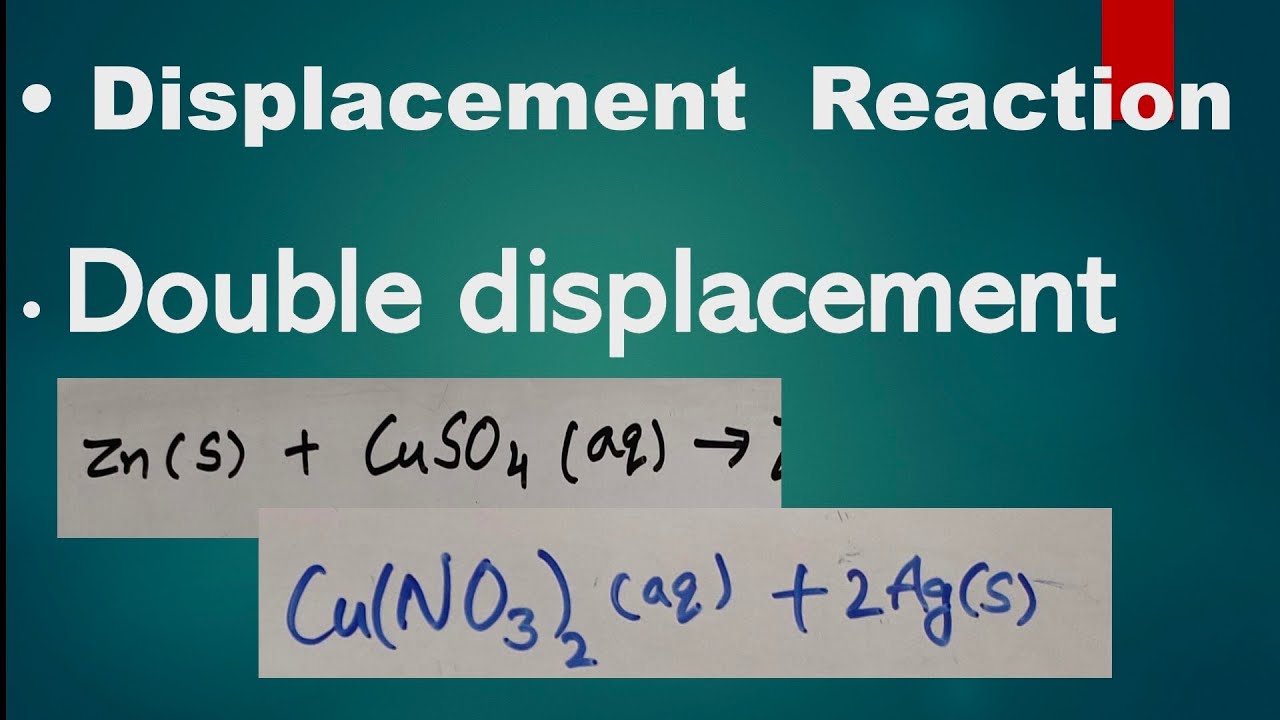
A Brief Look at Double Displacement Reactions
2.1 What Are Double Displacement Reactions?
Define double displacement reactions and distinguish them from other types of reactions. Highlight their unique characteristics, such as the exchange of ions between reactants.
2.2 Important Things About Double Displacement Reactions
Discuss the hallmarks of double displacement reactions, including the formation of a precipitate, the role of aqueous solutions, and the concept of spectator ions.
What makes double displacement reactions happen?
3.1 Ion Exchange: What Makes It Go
Explain how ion exchange is the driving force behind double displacement reactions, leading to the formation of new compounds.
3.2 How insoluble compounds form and why they do so
Detail the process of precipitation and the formation of insoluble compounds in double displacement reactions, with an emphasis on solubility rules.
Double Displacement Reactions: Some Examples
4.1 Reactions to Rainfall
Provide examples of precipitation reactions, showcasing the formation of insoluble precipitates.
4.2 Neutralization of Acids and Bases
Explore acid-base neutralization reactions as a subcategory of double displacement reactions, focusing on the production of water and salt.
4.3 Reactions That Make Gas
Discuss gas formation reactions, where the exchange of ions results in the release of gases.
4.4 Double-Displacement Reactions with Redox
Explain redox double displacement reactions, which involve both oxidation and reduction processes.
How Double Displacement Reactions Are Used
5.1 Processes in Industry
Highlight the role of double displacement reactions in industrial processes such as metal extraction and the production of chemicals.
5.2 Cleaning up the environment
Discuss how double displacement reactions contribute to environmental cleanup and treatment of pollutants.
5.3 Synthesis of Drugs and Chemicals
Examine their significance in pharmaceutical and chemical synthesis, particularly in forming specific compounds.
5.4 The Science of Analysis
Explore how double displacement reactions are employed in analytical techniques, including precipitation titrations.
What makes double displacement reactions happen?
6.1 Concentrations and Chemical Reactions
Explain how the concentration of reactants and the reactivity of ions impact the rate and extent of double displacement reactions.
6.2 Pressure and Temperature
Discuss the effects of temperature and pressure on reaction rates and equilibrium in double displacement reactions.
Methods of Experiments and Observations
7.1 Figuring out what double displacement reactions are
Outline methods for identifying double displacement reactions based on observable changes and characteristics.
7.2 Watching Rain and Snow Form
Detail techniques for observing and characterization of precipitate formation in double displacement reactions
When an acid reacts with a base, the pH changes.
Explain how pH changes can indicate the occurrence of acid-base neutralization reactions.
Common Misconceptions and Troubleshooting
8.1 Misinterpretations of Precipitation
Address common misconceptions related to precipitate formation, such as mistaking a clear solution for no reaction.
8.2 Balancing Redox Double Displacement Reactions
Provide strategies for balancing complex redox double displacement reactions, considering both oxidation and reduction processes.
Double Displacement Reactions in Everyday Life
9.1 Water Treatment and Softening
Explore how double displacement reactions are used in water treatment and softening processes.
9.2 Cooking and Food Chemistry
Discuss their role in cooking and food chemistry, including the preparation of various dishes.
9.3 Cleaning Agents and Household Products
Examine how double displacement reactions contribute to the effectiveness of cleaning agents and other household products.
Educational Importance and Teaching Approaches
10.1 Enhancing Chemical Education
Highlight the educational significance of double displacement reactions in teaching foundational chemistry concepts.
10.2 Hands-on Demonstrations and Experiments
Offer practical suggestions for conducting hands-on demonstrations and experiments to teach double displacement reactions effectively.
Challenges and Future Perspectives
11.1 Complex Reaction Systems
Discuss the challenges associated with complex reaction systems involving multiple reactants and products.
11.2 Cutting-Edge Applications in Nanotechnology
Explore emerging applications of double displacement reactions in nanotechnology and advanced materials.
Conclusion
Double displacement reactions play a vital role in understanding the interactions between substances and are essential in various scientific, industrial, and everyday contexts. By comprehensively exploring their mechanisms, examples, applications, and educational significance, this guide underscores the fundamental importance of double displacement reactions in the realm of chemistry. As we continue to unravel the intricacies of chemical reactions, the study of double displacement reactions remains a cornerstone in our journey of discovery and innovation.